28/01/2026
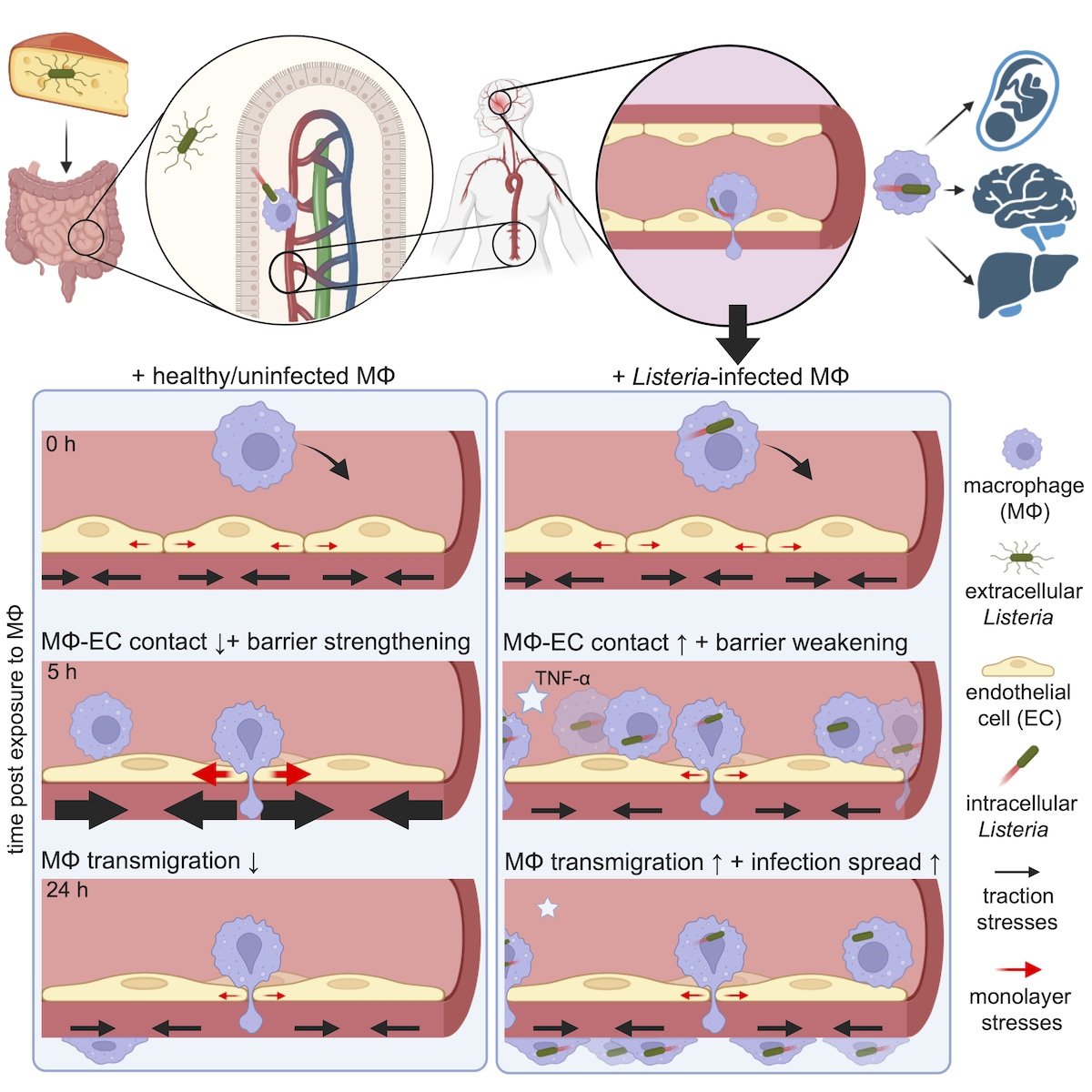
🎉 Congratulations to Marie Münkel and co-authors on getting our work entitled “Listeria-infected macrophages promote biomechanical alterations in endothelial cell monolayers for transmigration” accepted for publication at Cell Reports.
Intracellular pathogens like Listeria exploit macrophages to cross endothelial barriers and spread systemically. Muenkel et al. show that exposure to infected macrophages weakens contractile forces within the endothelial monolayer and promotes macrophage transmigration. This response is driven by direct cell–cell interactions, with cytokines exerting only minor and transient effects. More details on this work as soon as article in in press. (Preprint, DOI: 10.2139/ssrn.5357421)
15/11/2025
🎉 Congratulations to Marie Münkel and Raúl Aparicio-Yuste!
We are delighted to congratulate Marie Münkel on successfully defending her PhD on 22.10.2026 with an outstanding magna cum laude. We are incredibly proud of her achievements and wish her all the best as she begins her postdoctoral fellowship in Florian Wimmers’ lab. We look forward to seeing the exciting science she will undoubtedly accomplish!
We also warmly congratulate Raúl Aparicio-Yuste, postdoctoral researcher in the Bastounis Lab, for being awarded the prestigious two-year Research Fellowship of the Alexander von Humboldt Foundation. This highly competitive award recognizes his excellent scientific accomplishments and the promise of his future work. We are thrilled for him and proud to have him as part of our team.
Bravo to both Marie and Raúl!
04/11/2025
We are delighted to announce the Mechanobiology of Infections Conference to be held in Ascona, Switzerland, from June 7 to 11, 2026: https://www.biophysics.org/meetings-events/thematic-meetings/2026-ascona-thematic-meeting
This will be the first international conference dedicated to understanding how mechanical forces shape microbial physiology and infection. The meeting will explore how tissue mechanics influence bacterial behavior, virulence and immune responses, highlighting a new dimension at the intersection of microbiology and mechanobiology. The program will feature invited talks, contributed presentations and poster sessions in the amazing venue of Monte Verità in Ascona. Feel free to circulate this announcement among your groups and beyond. We look forward to seeing you in Ascona!
The organizing Committee: Effie Bastounis, Daria Bonazzi, Alex Persat
09/05/2025
We are inviting applications for a PhD position (E13 TV-L, 65%) at CMFI - Cluster of Excellence within the Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen. This position is part of a Deutsche Forschungsgemeinschaft (DFG) - German Research Foundation-funded collaborative project (2nd funding phase) in partnership with the Iordania Constantinou lab at Technische Universität Braunschweig. The project investigates how mechanical forces shape the dynamics of intracellular bacterial infections in epithelial tissues, focusing on the food-borne pathogen Listeria monocytogenes. For detailed information on candidate profile, responsibilities, and the application process, please visit: https://lnkd.in/eyjxwTzS. Start Date: As soon as possible!
09/05/2025
We are happy to share that Felix Romer submitted his master’s thesis last week:
“Heterotypic Interactions Between Endothelial Cells and (Infected) Macrophages: Characterisation of Endothelial Cell Shape Alterations, Organisation, and Macrophage Adhesion”
In this collaborative project with Prof. Dr. Peter Loskill’s group, Felix investigated how Listeria monocytogenes-infected and uninfected macrophages influence endothelial cell alignment and morphology in microfluidic channels exposed to shear stress.
09/05/2025
Thrilled to have contributed data — originally generated years ago during my time in Rick Firtel’s lab at UC San Diego— to a project led by Calina Copos’ group at Northeastern University.
The study, focused on the mathematical modeling of how cell chains (or trains, or streams) migrate, has just been accepted for publication in npj Systems Biology and Applications!
📄 Title: “Emergence of multiple collective motility modes in a physical model of cell chains”
09/05/2025
A few months ago, Lara Hundsdorfer et al. published an exciting study on how ERK waves coordinate mechanical cell competition during intracellular bacterial infection [read it here].
Now, we’re happy to share a follow-up: a detailed STAR Protocol, co-authored by Julio Sanchez-Rendon and Lara H., describing how to characterize cell motility and ERK dynamics in epithelial monolayers using FRET imaging data — hopefully a powerful tool for the community that wants to characterize propagating signaling waves in monolayers! [check it out here]
02/02/2025
🎉 Exciting news from the lab! Three new papers are out!
Aparicio-Yuste et al. presents a powerful hybrid computational model to simulate host-pathogen biomechanics — now published in Computers in Biology and Medicine [more here].
Our new review in Trends in Cell Biology discusses how modeling human cell monolayers contributes in shedding light onto cell and tissue biomechanics. [more here].
Jaworski et al., in collaboration with the Constantinou lab at TU Braunschweig, showcase our custom-designed cell-stretching device that works seamlessly with time-lapse videomicroscopy — now out in Advanced Science [more here]
06/02/2024
Excited that our DFG grant on vascular infections got funded! We are looking for a PhD student to study the mechanobiological processes involved in direct infection of vascular endothelial cells by food-borne facultative intracellular bacterial pathogen Listeria monocytogenes. If you would like to join the University of Tübingen and the cluster of excellence “Controlling Microbes to Fight Infections” (CMFI) as a doctoral student please contact me. More details about applying can be found here.
22/09/2023:
Effie was elected International Fellow of the American Heart Association (FAHA) conferred by the Council on Arteriosclerosis, Thrombosis and Vascular Biology (ATVB). This fellowship recognizes and awards professional AHA members for excellence, innovative and sustained contributions in the areas of scholarship, practice and/or education, and volunteer service within the AHA/ASA. We are grateful to AHA about this honor!
15/09/2023:
The German Research Foundation (DFG) approved our grant for the purchase of a spinning disk confocal microscope (SDCM)! Together with professors Alex Weber, Peter Loskill, Heike Broth-Oesterhelt and Samuel Wagner and with the financial support of DFG and Universitätsklinikum Tübingen, we will soon acquire a SDCM system which we will be able to operate in a biosafety level 2 environment to observe the spatiotemporal dynamics of infection processes!
01/08/2023:
Graduate students Marie Muenkel and Raul Aparicio-Yuste have both been awarded graduate student grants! Marie got the EMBO scientific exchange grant and is currently visiting Serge Mostowy’s lab at the London School of Hygiene & Tropical Medicine and will be back with us in November! Raul got awarded a Complementary Mobility Grant from the government of Spain which is funding his research stay in Tuebingen from March to October 2023!
14/06/2023
Congrats to Lara Hundsdorfer for the great talk on ERK waves during intracellular bacterial infection of epithelia & Marie Muenkel for winning the best poster award on how infected macrophages interact with endothelia @ the EMBO/EMBL Symposium 2023 - Life at the periphery: mechanobiology of the cell surface!
28/03/2023
It was exciting to attend the 3rd BioMechBW at the University of Stuttgart! Great talks Lara Hundsdorfer & Julio Sanchez!
Thank you Andrew Clark & Michael Heymann for hosting the 3rd BioMechBW biomechanics workshop at the University of Stuttgart & giving us the opportunity to present our work & discuss new ideas. Thanks also to Lennart Hillbert, Raphael Reuten and Linus Stegbauer for the great talks! Looking forward to the 4th BioMechBW biomechanics workshop at the University of Tübingen, in fall 2023
12/01/2023
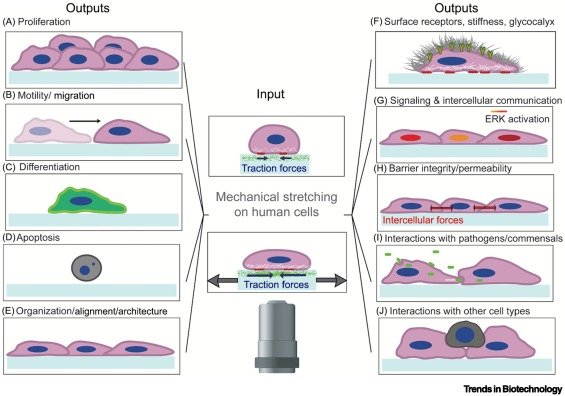
Our review on cell stretching devices during live-cell imaging is accepted in Trends in Biotechnology!
Interested in how cell stretching devices during live-cell imaging can advance biomedical research? Check our review paper just published in Trends in Biotechnology, where we discuss recent exciting developments in the field here: https://doi.org/10.1016/j.tibtech.2022.12.009.
11/11/2022
Our protocol paper is published in STAR Protocols!
Check our protocol paper just published in STAR protocols detailing how to expose endothelial cells to Lyme disease pathogen Borrelia burgdorferi and characterize changes in cell kinematics and dynamics here. Congrats Marie Muenkel and Raul Aparicio-Yuste for the great work> Also, special thanks to Kraiczy lab at University of Frankfurt & Michal Caspi Tal at MIT!
07/28/2022
Our paper on Borrelia burgdorferi interactions with endothelial cells is accepted in iScience!
Excited to share our study on how Borrelia burgdorferi (Bb), the causative agent of Lyme disease, modulates the physical forces and immunity signaling in endothelial cells (ECs) in vitro using live cell imaging! Read here.
Bb can spread to distant tissues in the human host by traveling in and through monolayers of endothelial cells (ECs) lining the vasculature. Through videomicroscopy of ECs exposed to Bb and transcriptomics, we discovered that the pathogen does so by concurrently and actively modulating the EC physical forces and innate immune signaling responses of host cells in a time-dependent manner, with ECs exhibiting strong biomechanical alterations at early but not late exposure to pathogens.!
07/07/2022
Our first paper is published!
Wonder how matrix stiffness impacts the collective extrusion of bacterially-infected cells? Read our recent publication: https://www.frontiersin.org/articles/10.3389/fcell.2022.912318/full! This was a great collaboration between Raul Aparicio-Yuste (modeler) and Marie Münkel (experimentalist) and Andrew Clark (microscopy resources). Thank you CMFI for the support! Also congrats to Marie for winning the best poster prize while presenting this work at the VAAM/DGHM Symposium on Microbial Pathogenicity !
17/03/2022
Our review on how mechanics drives infection processes now at MMBR!
Our review with co-authors Effie Bastounis, Prathima Radhakrishnan, Chris Prinz, and Julie Theriot (UW Biology) on how mechanical signals drive infection processes is now published in Microbiology and Molecular Biology Reviews ! Check it out here!
How do macrophages spread infection into underlying endothelia on an endothelium-on-chip device that allows exposed of cells to fluid flow?
15/11/2021
Collaborative research grant got funded & we are looking for a postdoc!
Our collaborative CMFI research grant got funded! The Bastounis and Loskill labs (http://www.loskill-lab.com) are looking for a highly motivated Postdoc to develop an endothelium-on-chip device and explore how bacterially-infected macrophages interact with endothelia when exposed to shear fluid flow. For more info check here. Deadline 20-12-21.
Biomechanics of bacterial infection spread in a multiplex cell stretching device that mimics intestinal peristalsis.
8/11/2021
Our DFG collaborative grant got funded and we are hiring!
We are very excited that our collaborative DFG grant got funded! The Bastounis lab and the Constantinou lab at TU Braunschweig are looking for two enthusiastic candidates interested to conduct their PhD on exploring how human intestinal cells interact with infectious bacteria in a novel stretching device that mimics intestinal peristalsis! This will be a highly collaborative work! Interested? Check here for more information.
9/15/2021
Two more students on board!
We are so excited to have Marie Münkel (PhD student) and Konstantinos Axarlis (Erasmus+ student) join out team! Konstantinos will study how biomechanical signals modulate transfer of bacteria from infected macrophages into endothelial cells when subendothelial stiffness varies, while Marie will focus on understanding how these bacteria-carrying macrophages squeeze themselves to transmigrate through endothelial cell monolayers and thus spread infection systemically!
7/16/2021
Let’s welcome Annalena Reuss and George Gkekas in our group!
Let’s welcome on board Annalena Reuss and George Gkekas, both PhD students in Biology! Annalena will be studying how shear stresses due to fluid flow impact bacterial infection spread in endothelial cells. George is wondering how epithelial cells at late infection with intracellular bacterial pathogens get collectively extruded. He plans to investigate the spatiotemporal modulation of biochemical signals during the mechanical battle of infected versus surrounding uninfected cells!
5/25/2021
Volume measurement & biophysical characterization of mounds in epithelial monolayers after intracellular bacterial infection
Are you interested in performing biophysical measurements on bacterially-infected epithelial cells in monolayer? In our recently published work at STAR Protocols we describe all experimental steps and computational workflow for (1) measuring volume of extruded infected cell domains and (2) characterizing indirectly the tension build between infected and uninfected cells in monolayer through a laser wounding approach. Follow this link for more details.

Open positions
We are seeking graduate students and postdoctoral researcher to work in the intersection of cell biomechanics and host-pathogen interactions.

Check our paper in Developmental Cell on infection mounds
Mechanical battle between cells leads to clearance of bacterially infected cell domains .